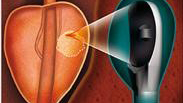
A public-private collaboration in Lyon, France, is underway to develop an alternative therapy for standard prostate cancer treatment. The clinical trial phase of “PERFUSE” recently began enrolling patients, an important milestone in this multi-faceted project. The clinical trial is using EDAP-TMS’s Focal One prostate device for a multicenter, randomized study that compares high-intensity focused ultrasound (HIFU) focal treatment to active surveillance in low-risk patients. Furthermore, a preliminary cohort study will evaluate focal HIFU mid-term results in intermediate-risk patients. A third cohort study will assess focal HIFU in patients with local recurrence after radiotherapy. Patients will be selected by a prostate-specific membrane antigen (PSMA)-based imaging modality, positron emission tomography (PET)/MRI. The treatment will then be guided by PSMA-PET/MRI imaging and performed with a hybrid device. A fourth ancillary study will enroll intermediate-risk patients and patients with local recurrence to assess whether the HIFU procedure induces an immune response and whether circulating tumor cells and/or prostate cancer antigen 3 cells (CTC and PCA3) can be used as biomarkers for disease tracking.
The PERFUSE study is a University Hospital Research project funded by the French National Research Agency. It is a partnership between the academic centers conducting the study, which include: the Laboratory for Therapeutic Applications of Ultrasound (LabTAU); the Biomedical Imaging Research Laboratory (Centre de Recherche en Acquisition et Traitement de l’Image pour la Santé or CREATIS); the Lyon Cancer Center (Centre Leon Berard or CLB) and the University Hospitals of Lyon (Hospices Civil de Lyon or HCL); and two international companies: EDAP and Vermon.
“This project aims to improve the treatment of prostate cancer through targeted ablation of the tumor by HIFU,” said Principal Investigator Prof. Sébastien Crouzet.
As an alternative to standard prostate cancer therapies, HIFU focal treatment targets only the area affected by the cancer and leaves the remainder of the prostate intact, which dramatically reduces the risks of impotence and incontinence. Focal One is a noninvasive treatment that combines ultrasound-MRI fused imaging, focused ultrasound energy with proven efficacy, and immediate post-treatment contrast enhanced ultrasound imaging to confirm ablation in the targeted area.
This project will provide an updated clinical evaluation of the focal HIFU treatment applied to localized cancers and aims to improve imaging and therapy performance in order to ultimately improve focal treatment outcomes.
In parallel to the clinical trials, scientists also plan on further developments, including 1) a computer-assisted diagnostic (CAD) system for prostate MRI to provide probability maps that highlight cancerous areas; 2) new imaging methods (MR/US elastography) for improved tissue characterization and treatment planning; 3) novel F-HIFU energy delivery and new treatment planning strategies; and 4) a new type of dual-mode planar transducer for high-resolution imaging and therapy using capacitive micromachined ultrasonic transducers (CMUTs).
