
Shenzhen PRO HITU Medical Co., LTD., is an international, high-tech Chinese research and development company founded more than 15 years ago that builds ultrasound-guided focused ultrasound systems. Initially launching their devices in the treatment of uterine fibroids, the company is looking toward a future of treating other types of tumors, such as those that cause pancreatic cancer. We recently interviewed Pro-HITU’s co-founder and CEO, George Zhang, to learn more.
How was the company started?
Pro-HITU was founded in 2003 when the high-intensity focused ultrasound (HIFU) treatment concept was still new and only a few companies existed in this field in China — a country with a remarkable potential market due to its large population. China’s high population and unmet need for treating women with uterine fibroids motivated us to establish Shenzhen PRO HITU Medical Co., LTD, for developing and manufacturing large ultrasonic treatment equipment. Our vision is to be pioneers of noninvasive therapy, and our mission is to “Respect Life in Therapy.”
How did you get involved in starting or joining the company?
I am the co-founder of this company with two other friends. My major in university was biomedical engineering, so I learned about HIFU technology more than 20 years ago. After graduation, my work experience was in Research & Development (R&D) and Sales and Marketing of medical equipment, mostly image systems including type-B ultrasound, CT, and MRT. So, believing in HIFU’s advantage of noninvasive treatment in solid tumor, we set up the company, starting with R&D of equipment for myoma and adenomyosis treatment.
 Tell us about your company structure: ownership, lead executives, and their roles.
Tell us about your company structure: ownership, lead executives, and their roles.
There are around 100 employees in our company, so we have complete structure and roles. As a founder, I am also CEO, responsible for partner cooperation, financing, strategy, and product planning. The lead executives include:
- The general manager, who is responsible for the daily operation and management of the company and is in charge of sales and marketing.
- A deputy general manager, who is also the secretary of the board and is responsible for the operation of the quality system and the quality control.
- The Director of R&D, who is responsible for new product development, existing product improvement, patents application, and product registration.
- The Director of Production, who is responsible for production, procurement, warehouse, and after-sales service.
- Our clinical department is responsible for clinical experiments in hospital and training the doctors how to use our equipment after hospital installation.
- We also have a human resources manager, a finance manager, a marketing manager, and sales managers in major districts.
In general, what is the current status of your company?
Our company is a national high-tech enterprise that integrates R&D, production, sales, and investment services. We are the leading company in HIFU segmentation in China in terms of advanced technology and market share.
There have been more than 100 units of our PRO equipment installed in hospitals in China. We have also exported nine units to Korea and two units to Taiwan.
We are currently developing new products for therioma (cancer), such as pancreatic cancer, while improving our existing gynecological treatment equipment to make it more user friendly.
In the aspect of financing, we are in the process of raising a third round of funding before IPO.
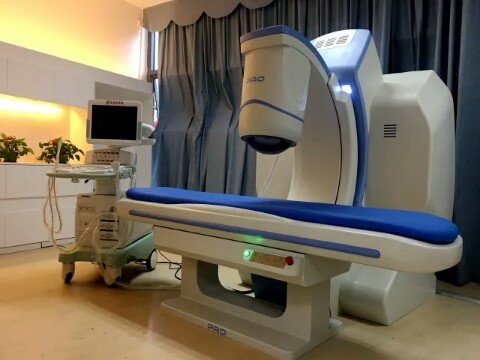 How many years has your treatment platform been in development, and what are its origins? Does it have a name?
How many years has your treatment platform been in development, and what are its origins? Does it have a name?
Our first product, PRO2008, started development in 2004, underwent clinical trials from 2007, and then obtained Chinese Food and Drug Administration (CFDA) approval in February 2012. It was the first CFDA-certified HIFU system for gynecological treatment. PRO 2008 then obtained the CE Mark in June 2012.
The upgraded PRO300 (which was based on the PRO2008) obtained CFDA approval in 2018.
How is your system being used in these markets?
As mentioned above, more than 100 of our PRO equipment units have been installed in hospitals in China and other Asian countries. The system is guided and monitored by type-B ultrasonic images, and the ultrasound is focused on the target area to form a focal region. The dense ultrasonic energy in the focal region causes the temperature to rise rapidly in a short time, which leads to coagulative necrosis ablation.
Ultrasonic or MRI contrast evaluation have proven that the uterine fibroids gradually shrink and disappear after the inactivation, and that the treatment does not harm the normal tissue. The high temperature produced by the treatment can also destroy the hormone receptor on the cell membrane of the myoma, thus reducing its sensitivity to hormones, which can prevent the regeneration and enlargement of uterine myoma. For these reasons, we believe that HIFU is the best method for early treatment and repeated treatment of uterine myoma.
What are some of the technical challenges your group has to overcome to develop a fully noninvasive system?
We have worked to improve the performance of energy conversion and platform stability while attaining clinical verification of the curative effect. We have also completed evaluation of ablation and dose control while creating standard operating procedures (SOP) for using the device.
What challenges do you have to tackle moving forward?
Our current challenges are to obtain clinical experts’ acceptance of HIFU as an innovative technology and to combine noninvasive treatment with traditional treatment to provide a more comprehensive solution for patients.
Which health conditions or diseases will your technology be used for?
The noninvasive advantages of “no pain, fast recovery, low risk, short time” make HIFU a new treatment for many gynecological diseases, including myoma and adenomyosis. The myoma is the most common benign tumor in women of childbearing age. Medical therapy is not effective enough, and traditional surgery can damage the human body. The effectiveness and feasibility of HIFU treatment for myoma and adenomyosis have been confirmed by a large number of clinical studies and practices worldwide. HIFU is the best method for early and repeated treatment of myoma and adenomyosis. Our system is used in the treatment or auxiliary treatment of myoma , adenomyosis, placental implantation, scar pregnancy, and more. We are also developing new products for treating therioma (e.g., pancreatic cancer).
What are the benefits of your technology over companies?
Circular Self-focusing Ultrasonic Transducer
– Precise focal spot, with a minimum of 1.1 x 1.1 x 3.3 mm
– Well-distributed ultrasound pathway with sufficient HIFU dose
– Optimized focusing mechanism that minimizes the risk of damaging the tissue in the ultrasound pathway
Fixed Safe Depth Range for Gynecology Treatment
– A preset maximum treatment depth to ensure uterine safety
– The system’s range of focal depth prevents damage to the tissue and nerves below the uterus
Top-mounted Treatment Head
– The overhead design allows patients to comfortably lie flat during the procedure (other companies’ designs require patients to lie prostrate)
– The coupling water does not directly contact the patients, which delivers a better treatment experience
C-Arm Design
– The flexible positioning mechanism provides the optimized ultrasound pathway
Treatment Planning System
– Precisely locates the fibroids
– Quickly finalizes the treatment plan with its conformal function
– Dynamically displays, records, and tracks the treatment using ultrasound imaging
Short Treatment Time
– Type-B ultrasonic image guidance makes it possible to complete the treatment within 30 to 45 minutes, compared to the longer time required for MRI guidance
Tell us about your clinical studies and the results.
We have conducted clinical cooperation with many prestigious hospitals, including Xiangya Hospital of Central South University, Peking University People’s Hospital, the Affiliated Hospital of Zhejiang University, the Affiliated Hospital of Jinan University, and Renji Hospital of Shanghai Jiaotong University. Positive results have been achieved.
Have you learned any lessons for watching the experience of the other companies?
Customer and consumer research are always the first steps. We need to identify the real needs and the pain points and then develop the product as a solution. Technology is key, and we have to make the product effective, stable, and safe. Marketing is also very important because HIFU is still new and not widely accepted by doctors in hospitals. Therefore, we need to educate doctors about the concept of HIFU and its effectiveness and benefits.
Do you partner with other companies?
We partner with other companies in innovative technology (e.g., the direct cavitation effect). We also have extensive collaboration with outside research teams, such as the Chinese Academy of Science, Shanghai Jiaotong University, Tongji Unversity, Shenzhen University, and more. Finally, we partner with distributors in Korea and Taiwan.
Is there anything else we should know about your company?
Our company has always insisted on innovation. We are now developing new ultrasonic treatment equipment for other tumors (including pancreatic cancer and prostate cancer) and chronic pain.
Pro-HITU has filed dozens of technology patents and earned several qualifications, including being designated a “National High-tech Enterprise.” We have also gained many laurels, including receiving the “Shenzhen Green Medical Technology Innovation Award,” and winning the “Champion of China (Shenzhen) Innovation and Entrepreneurship Competition.”
We are committed to bringing noninvasive ultrasound therapy into broader medical application fields.
