Researchers at Sunnybrook Health Sciences Centre have published data from the first-in-the-world clinical trial to open the blood-brain barrier (BBB) in patients with glioblastoma (GBM).
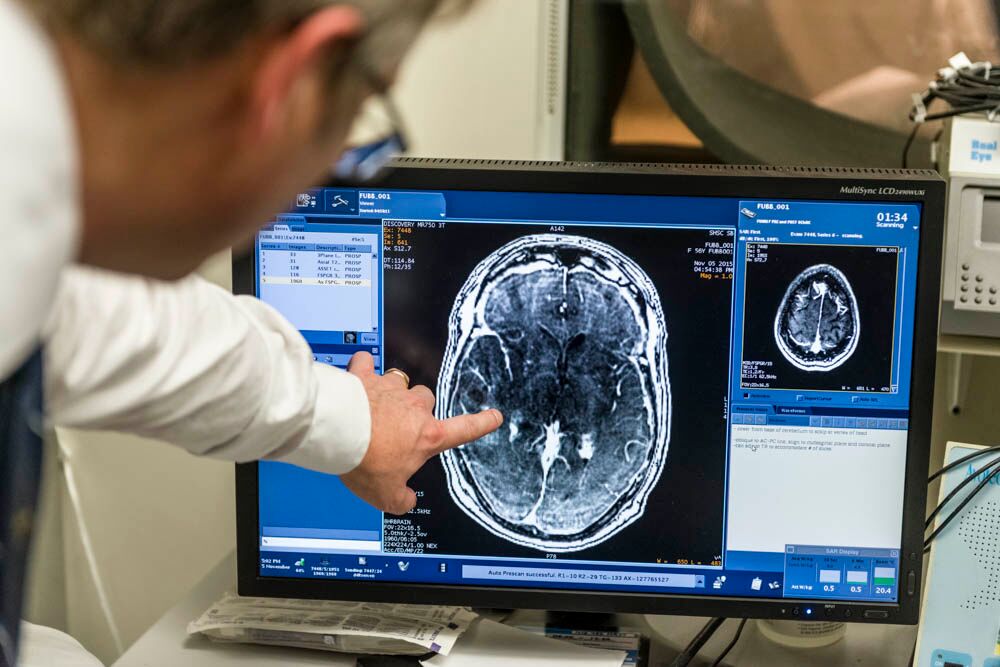 In “Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study,” the Sunnybrook team described how they successfully used focused ultrasound plus microbubbles to temporarily and repeatedly disrupt the BBB in a targeted fashion, without open surgery. Five patients with previously confirmed or suspected high-grade glioma based on imaging underwent BBB opening while receiving chemotherapy the day prior to surgical resection of the tumor. The procedure proved to be safe and feasible in this phase I, single-arm, open-label study. The team was also able to measure increased levels of drug delivery in parts of the tumor that were sonicated.
In “Blood-Brain Barrier Opening in Primary Brain Tumors with Non-invasive MR-Guided Focused Ultrasound: A Clinical Safety and Feasibility Study,” the Sunnybrook team described how they successfully used focused ultrasound plus microbubbles to temporarily and repeatedly disrupt the BBB in a targeted fashion, without open surgery. Five patients with previously confirmed or suspected high-grade glioma based on imaging underwent BBB opening while receiving chemotherapy the day prior to surgical resection of the tumor. The procedure proved to be safe and feasible in this phase I, single-arm, open-label study. The team was also able to measure increased levels of drug delivery in parts of the tumor that were sonicated.
We reported on the first patient treated in this trial, which also marked the first time the BBB was opened noninasively using focused ultrasound in a clinical setting.
See the paper in Scientific Reports >
