
U.S. start-up HistoSonics, Inc. is preparing to launch clinical trials using its noninvasive pulsed ultrasound technology to treat benign prostatic hyperplasia (BPH), an age-related condition that, over time, affects nearly all men. Estimates indicate that 50% of men over 50 and 80% of men over 80 have BPH. More than two million Americans have the condition and about 400,000 are treated surgically for BPH each year.
HistoSonics (a combination of histo meaning tissue and sonicsmeaning sound waves) intends to eliminate that shortfall by giving urologists a new, noninvasive option – histotripsy. Invented by scientists in the Biomedical Engineering and Urology Departments at the University of Michigan, histotripsy represents an entirely new use of focused ultrasound energy. The current spectrum of care for BPH begins with watchful waiting when symptoms are mild. As symptoms worsen, treatment progresses to drug therapy, minimally invasive outpatient procedures and/or surgery. Today’s gold standard of care is transurethral resection of the prostate (TURP), a surgical procedure that is performed in several ways. “While surgical alternatives have better long-term outcomes than minimally invasive procedures, they can result in complications like bleeding, incontinence and impotence,” says HistoSonics President and Chief Operating Officer M. Christine Gibbons. “These treatment alternatives fall short in one way or another.”
While most of today’s ultrasound procedures use heat to destroy diseased or unwanted tissue, histotripsy takes another approach. It employs a bio-mechanism called acoustic cavitation. During histotripsy, pulses of focused ultrasound induce microbubbles to form and collapse (cavitate) causing targeted tissue to liquify. The process involves temperatures too low to cause burning.
“Ablated tissue is homogenized into a fluid, with no cellular content, that is rapidly voided or absorbed leaving little scarring or remnant,” Gibbons explains. “Histotripsy can be controlled to exact treatment margins and can more safely and precisely ablate tumors that are adjacent to vital structures such as vessels and nerves. It has the potential to replace open and minimally invasive procedures.”
The Vortx RX Histotripsy System
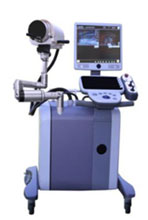
HistoSonics has identified BPH as the first indication for its Vortx RX Histotripsy System.
HistoSonics has completed preclinical studies and developed, ed and verified its first technology platform, the Vortx RX. Regulatory and Institutional Review Board (IRB) applications are pending, and BPH clinical trials are expected to begin later this year. In addition to histotripsy technology, the Vortx RXincorporates ultrasound imaging for pre-operative treatment planning and for real-time treatment monitoring and verification. It uses proprietary software for procedure planning and tracking.
According to Gibbons, histotripsy treatments of BPH will be performed on an outpatient basis in the offices of urologists. Procedures will begin with a 3-D image reconstruction of the prostate. The treating urologist will then create a 3-D outline of the target tissue volume to be ablated.
“After completion of the procedure plan, the urologist steers the cavitation field, which appears as a bubble cloud on the ultrasound image, through the target volume using joysticks with precise robotic control,” she explains. “The urologist monitors the procedure in real time with ultrasound image guidance.”
Future applications, current challenges
The Vortx RX is designed to be a versatile technology platform used for various indications. “Preclinical studies conducted by University of Michigan have verified potential applications in tumor ablation, fetal heart surgery, thrombolysis, lithotripsy and others,” Gibbons notes.
A key challenge facing HistoSonics is the need to focus on BPH while continuing to prioritize the next application(s) of histotripsy and balancing resources to pursue future possibilities. “We are open to partnering or other opportunities to allow additional applications of histotripsy to advance in parallel,” she says.
Another challenge is educating the research and clinical communities. As Gibbons notes, “The beneficial attributes of histotripsy are sometimes misunderstood, given its unique characteristics and frequent confusion with HIFU.”
Company background
Based in Ann Arbor, Michigan, HistoSonics is a privately held company launched in December 2009 following completion of an $11 million round of Series A funding. Financial backers include Venture Investors, Fletcher Spaght Ventures, Hatteras Venture Partners, Early Stage Partners and TGap Ventures.
In addition to Gibbons, key executives are Tom Davison, PhD, Chairman and Chief Executive Officer and Jim Bertolina, PhD, Vice President of Research & Development and Chief Technology Officer.
The company founders and co-inventors of histotripsy are Charles Cain, PhD, Brian Fowlkes, PhD, Tim Hall, PhD, Zhen Xu, PhD and William Roberts, MD, all from the University of Michigan.
- VIEW HISTOSONICS VENDOR PRESENTATION from the 2012 International Focused Ultrasound Symposium
Written by Ellen C., McKenna
